- Hygroscopicity
- Water content of the goods
- Sorption behavior
- Moisture sensitivity
Section 10.2.9 made it clear that moisture sensitivity and hygroscopicity are two different concepts. For instance, a product is sensitive to moisture if even slight water vapor adsorption rapidly causes severe changes, which occur even at low relative humidities.
For example, sucrose is only slightly hygroscopic (see Fig. 5, sorption isotherm 2) but quite moisture-sensitive, because adsorption of even a small amount of water vapor results in the flow moisture point being reached and thus in liquefaction.
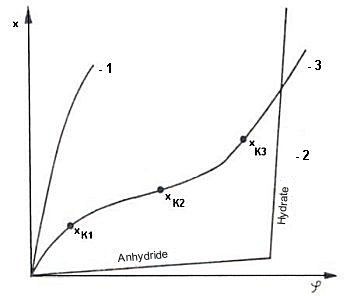 |
| Figure 5: Types of sorption isotherms Heiss [16] |
Steel goods are not at all hygroscopic, but are highly moisture-sensitive, i.e. susceptible to corrosion.
On the basis of these considerations, it is possible to divide goods along transport-meteorological lines into four main groups.
| 1. | Goods with a particular water content, which have the capacity to change constantly in accordance with the humidity and temperature conditions of the ambient medium. Such goods display undesirable changes due either to dampening (mold, rot, mildew stains, fermentation, deliquescence, self-heating) or to desiccation (solidification, jamming/caking, fragmentation, drying-out), depending on relative humidity and temperature. The sorption isotherms exhibit a continuous S-shaped profile without sudden discontinuities (see Fig. 58). These include: |
| 1.1 | the overwhelming majority of foodstuffs, which are characterized by mold, rot and fermentation on the one hand and by desiccation phenomena in conjunction with loss of aroma on the other (bread and bread products, pasta products, flour and also pharmaceuticals) |
| 1.2 | hygroscopic goods, which, as living organisms separated from the parent plant, may have a tendency towards self-heating as a result of respiration processes in addition to mold, rot or fermentation (cereals, seed, oil-bearing seeds/fruits) |
| 1.3 | hygroscopic goods which, as a result of processing methods, have a tendency, due to their residual oil content and/or critical water contents, towards self-heating and frequently towards spontaneous combustion (expeller, fish meal, cereal products) |
| 1.4 | hygroscopic goods which, during storage and transport, may be subject to postfermentation as a result of previous processing methods (fermentation), as well as to usual biochemical and physical changes (tea, green coffee beans, raw cocoa, leaf tobacco) |
| 1.5 | hygroscopic goods which have been preserved by drying and have a tendency towards syrup formation and fermentation (dried fruit, such as raisins, figs, dates) |
| 1.6 | hygroscopic goods which, in addition to mold and mildew staining, may also suffer loss in strength or splitting (packaging materials, such as paper, cardboard, fibers, fibrous materials and textiles, lumber and wooden articles) |
| 1.7 | Hygroscopic goods which, in addition to mold and mildew staining, may also suffer loss in strength or splitting (packaging materials, such as paper, cardboard, fibers, fibrous materials and textiles, lumber and wooden articles). |
| 1.8 | hygroscopic finished products, which are subject to the risk of deformation, splitting or loosening of glued joints when exposed to humidity/moisture and varying temperatures (furniture, musical instruments, craft items, toys) |
| 2. | Goods which may but do not necessarily contain water, any water which may be present being chemically bound. They do not react constantly to the moisture in the ambient atmosphere, but only after a certain specific threshold has been reached, the so-called flow moisture point. The adsorption isotherms are characterized by sudden discontinuities, of which there are as many as there are possibilities for hydrate formation. This class includes crystalline goods, which deliquesce (flow moisture point) after absorbing water vapor (water vapor adsorption) from the ambient air and may solidify and become jammed/caked (crystalline chemicals, fertilizer, salts, sugar) and harden (agglomeration) due to subsequent water vapor release. |
| 3. | Goods which do not contain any water and are incapable of absorbing water, but nonetheless suffer quality degradation or corrosion: |
| 3.1 | metals subject to corrosion, together with semifinished and finished articles without corrosion protection, including food cans |
| 3.2 | goods with a sensitive surface (optical products; clouding of lenses, sheet glass) |
| 4. | Goods which do not contain any water and are incapable of absorbing water, suffering no quality degradation due to humidity/moisture (certain plastics, industrial ceramic fittings, tiles etc.). However, losses of these goods may occur if the packaging is damaged by moisture and no longer meets transport requirements. This list, which may be used to classify further product groups, is also relevant to the question of fitness for container transport. Important data relating to humidity/moisture are given for each product, with appropriate references, on the cargo information pages, for instance relative humidity, water content and upper equilibrium moisture content. |
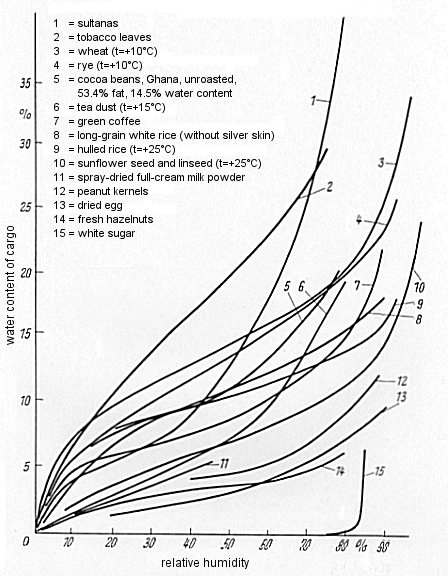 |
| Figure 58: Sorption isotherms for various goods [27] |