In a closed container, as a function of the water content of the substance, the relative humidity indicated by the sorption isotherm is established until an equilibrium between the product and ambient air is reached. This is known as the equilibrium moisture content. A graphical plot of the equilibrium states between the product partial pressure, which is dependent upon the water content, and the ambient air for a specific temperature produces the sorption isotherm, which consequently describes a product's sorption behavior. If a product's water content is known, it is possible to use the sorption isotherm to determine how the product will behave in the container or how the storage conditions or cryptoclimate will vary.
When determining the equilibrium states between the product and the ambient air, differences are found between the values which are measured during water vapor adsorption (adsorption isotherm) and those which are measured during water vapor release (desorption isotherm). The values for the desorption isotherm are always somewhat higher than those for the adsorption isotherm. The differences between adsorption and desorption isotherms are at their greatest at moderate relative humidities (Fig. 3). The adsorption isotherm at 20°C, which describes the state of hygroscopic goods after manufacture, is generally used in practice.
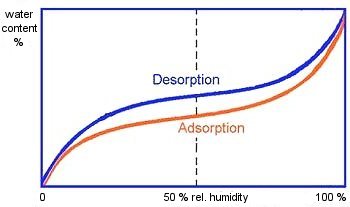 |
Figure 3: Adsorption and desorption curve; Tietke [58] Y-axis: water content X-axis: relative humidity |
The profile of a sorption isotherm is characteristic of the hygroscopicity of a product. Highly hygroscopic substances exhibit a steep sorption isotherm, while sparingly hygroscopic goods exhibit flat sorption isotherms. Weakly hygroscopic goods exhibit no or only a slight change in their water content as a consequence of variations in relative humidity.