The membrane itself consists of a polymer that is permeable to different degrees for different gases. In principle, almost all gases can pass through the membrane, but at different permeation rates. For the gases involved in CA applications, the following applies: Water vapor permeates fastest, followed by CO2, O2 and finally N2. The ratio between the permeation rates of two gases is known as selectivity. The driving force for the exchange of gases is the partial pressure difference of the gases on either side of the membrane.
This effect is exploited in nitrogen separation, in that oxygen can be extracted from compressed air.
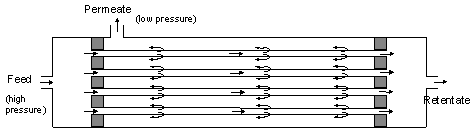 |
| Figure 52: Fundamental structure of a gas separation membrane |
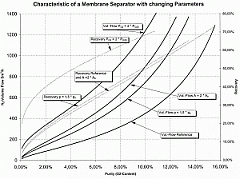
Furthermore, higher membrane temperatures increase the degree of permeability, so that today, membranes are generally used at as high a temperature as possible in order to achieve the greatest possible yield from a small membrane. At the same time, the selectivity between oxygen and nitrogen drops as the temperature increases, i.e. the efficiency is reduced in relation to the volume of compressed air used. This effect is not significant with membranes which have a high selectivity to start with. If, however, the selectivity of a membrane is limited, it does not make sense to operate it at high temperatures.
Today, membrane systems are generally used if residual oxygen content of 1% is adequate. Greater degrees of purity can be generated more cost-effectively with PSA systems. For CA in refrigerated containers, membrane systems are now used almost exclusively, because they are based on a simple principle and can be constructed to be light and compact. They consist of the following key components (see Figure 54):
- Air compressor with a suction filter
- Filter and water extractor in the compressed air supply
- Heating component to heat the compressed air
- Gas separation membrane
- Choke point to reduce pressure
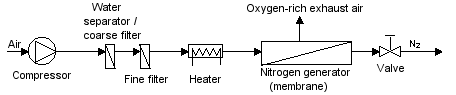 |
| Figure 54: Basic principle of a membrane type nitrogen separator |